Chemical Bonds
Bonds
Force keeping atoms and molecules together is called bond . Atoms come together and becomes more stable and energy is released during this process. Thus, we can say that all bonding reactions are exothermic. On the contrary, all breaking bonds reactions are endothermic. Valence electrons of atoms and molecules play role in bonding. If bond binds atoms together, then we call it chemical bond . However, if bond bind molecules together, we call it molecular bond .
Chemical Bonds:
There are two types of chemical bonds;
- Ionic bond
- Covalent bond
While elements form compounds they tend to have electron configuration of noble gases. Except from He, all noble gases end their electron configuration with ns2 np6. In other words all shells of noble gases are filled. They are too stable. Atoms also want to be stable and complete their number of valence electrons to 8.
Lewis Structures of Atoms
Representations of the valence electron around symbol of elements with dots. For example;
11Na=1s22s22p63s1
As you can see Na has one valence electron in its outermost shell. We show it with Lewis formula;
Na●
On the other if 1s, 2s and 2p orbitals are full, then they are not represented with Lewis formula.
Example: 17Cl write Lewis formula of Cl atom.
17Cl=1s22s22p63s23p5
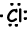
Valence electrons of ions can also be represented with Lewis formula. For example;
Lewis formula of 9F-1 is;
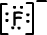
1. Ionic Bond:
It is the bond between positively and negatively charged ions. Metals and nonmetal atoms join together with ionic bond. Metal atom lose electron and becomes positively charged and nonmetal atom accept electron and becomes negatively charged. Force keeping ions together is electrostatic attractive force.
- In periodic table A group metals lose electrons equal to their group number. For example; metals in I A lose 1 electron and becomes +1 ion, metals in II A lose 2 electrons and becomes +2 ion, metals in III A lose 3 electrons and becomes +3 ion.
- Nonmetals accept electron that completes its valence electrons to noble gases. For example; nonmetals in V A group accept 3 electrons and becomes -3 ion, nonmetals in VI A group accept 2 electrons and becomes -2 ion, nonmetals in VII A group accept 1 electron and becomes -1 ion.
- During ionic bonding process, number of accepted electrons is equal to number of lost electrons.
Example: Analyze bond between NaCl molecule.
11Na loses 1 electron and becomes Na+. 17Cl accepts one electron and becomes Cl-. Attraction between opposite ions form ionic bond.
- Strength of ionic bond is directly related to tendency of losing electron of metals and accepting electron of nonmetals.
2. Covalent Bond:
If atoms share their valence electrons during bonding process, we call it covalent bond. There is no electron transfer. This type of bond is seen in between two or more nonmetal atoms. To have covalent bond, atoms must have at least one half filled orbital. Covalent bond between H2 molecule is shown below;
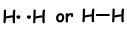
- Number of covalent bond is equal to number of half filled orbitals.
- First covalent bond between two atoms is called sigma bond and showed with “σ”.
- There is only one sigma bond between two atoms and other bonds are called pi bonds and showed with “π”.
Example: Analyze bond between O2 molecule.
8O=1s22s22p4
Or showing with orbital and Lewis dot schema;

As you can see from orbital schema and Lewis dot formula O has two half filled orbitals and it can does two bond. First bond is called sigma and showed below;
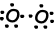
These O atoms share their second electrons and becomes O2 molecule;
Example: Show covalent bonds of NH3.
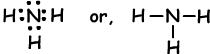
Example: Which one of the following statements is false for 8O element.
I. It is nonmetal
II. It can does two bonds
III. In ground state electron configuration it has two filled orbital
IV. I does covalent bond with 9F element.
V. It does ionic bond with 11Na and forms compound ; Na2O
Solution:
8O has electron configuration in ground state;

I. Since number of valence electrons of 8O is 6, it is nonmetal. True
II. It has two half filled orbital, thus it can does two bonds. True
III. As you can see from orbital schema, 8O has 3 filled orbital in ground state. False
IV. 9F has electron configuration in ground state;
F: 1s22s22p5
F has 7 valence electron and so it is nonmetal. We have learned that two nonmetal atoms join with covalent bond. True
V. 11Na has electron configuration in ground state;
Na: 1s22s22p63s1
Na gives one electron and becomes Na+ and O accepts 2 electrons and becomes O-2. Thus, bond between them is ionic bond. True.