Chemical Bonds Cheat Sheet
Chemical Bonds Cheat Sheet
Bonds
Force keeping atoms and molecules together is called bond . If bond binds atoms together, then we call it chemical bond . However, if bond bind molecules together, we call it molecular bond .
There are two types of chemical bonds;
- Ionic bond
- Covalent bond
Representations of the valence electron around symbol of elements with dots. For example;
11Na=1s22s22p63s1
As you can see Na has one valence electron in its outermost shell. We show it with Lewis formula;
Na●
It is the bond between positively and negatively charged ions. Metals and nonmetal atoms join together with ionic bond. Metal atom lose electron and becomes positively charged and nonmetal atom accept electron and becomes negatively charged. Force keeping ions together is electrostatic attractive force.
If atoms share their valence electrons during bonding process, we call it covalent bond. There is no electron transfer. This type of bond is seen in between two or more nonmetal atoms. To have covalent bond, atoms must have at least one half filled orbital. Covalent bond between H2 molecule is shown below;
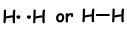
In bonds, forming between two same atom, electrons are attracted by equal forces. We call these bonds nonpolar covalent bonds . If covalent bond is formed between two different atoms having different electronegativity, then force acting on shared electron by the atoms becomes different. These types of bonds are called polar covalent bonds.
Hybridization and Bonding Geometry
We have learned that atoms can form bonds equal to number of half filled orbitals. On the contrary, when we look at molecule geometry or unexpected number of bonds of II A, III A and IV A groups, we explain it with another concept that is called hybridization.
sp hybridization
sp2 hybridization
sp3 hybridization
- linear
- trigonal planar
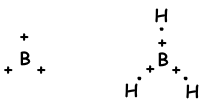
tetrahedral
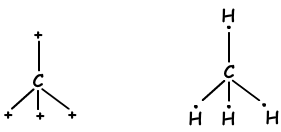
trigonal pyramidal
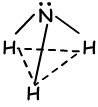
bent
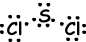
Bond that keeps metal atoms together is called metallic bond .
Atoms bonded with covalent bonds produce molecules and these molecules attract each other and form secondary bonds. We examine these bonds under three titles, Vander Waals Bond, Dipole-Dipole Interactions and Hydrogen Bond.
1) Van der Waals Bonds:
We see these bonds in molecules having % 100 nonpolar bonds like, I2, Cl2, H2 and molecules having polar bonds but nonpolar molecules like CO2. When nonpolar molecules like CO2 are get closer,they repel each other and symmetry of molecule is broken. And then, positive and negative dipoles are formed in molecule. An instant attraction of these dipoles each other is called Van der Waals bonds .
2) Dipole-Dipole Interaction:
This types of bond is seen between polar molecules in solid and liquid phases such as, HCl, SO2, H2S, PH3. Since there is no symmetry in polar molecules, there are poles having negative and positive charges. Attraction between these poles in molecule is called dipole-dipole interaction .
3) Hydrogen Bonds:
Chemical bonds formed between H and atoms having high electronegativity like F, N O, are stronger than dipole-dipole interactions. We can explain this ;
Since O has high electronegativity, it attracts H in H-O bond strongly and these bonds are called hydrogen bond. In other words, attraction between H atom of one molecule and O atom of another molecule is called hydrogen bond .
1) Ionic Solids:
2) Covalent Solids:
3) Molecular Solids:
4) Metallic Solids: